
Do geneticists encourage parents to have genetic testing or gene splicing to prevent inherited conditions?
April 30, 2014

- Related Topics:
- Bioethics,
- Genetic testing,
- Gene therapy,
- CRISPR
An undergraduate from Michigan asks:
"Do geneticists encourage gene splicing for people who can pass a genetic condition to their children? I guess what I am asking is if a parent would be required to undergo a genetic profiling before they are allowed to go ahead with their family planning? In so doing would the embryo be given a DNA scan? And should there be any deformity would that link be removed in order to create a "perfect" child?"
First off, gene splicing or editing like you describe currently isn’t possible for human embryos at all, let alone recommended! No one should ever make you change your baby’s DNA, and no medical professional ever will.
Secondly, no one requires anyone to receive genetic testing. This is a personal choice and shouldn’t be forced on anyone. And as for encouragement, that depends on the geneticist.
Right now, genetic testing can shed some light on what is lurking in your DNA. Unfortunately we don’t understand most of it and we can’t do much about the things we do understand. For example, we can’t safely or easily change “bad” DNA into “good.”
So while in the future we may be able to change the DNA in a human embryo, we can’t do this yet. The best we can do is look at the DNA in embryos and choose ones without genetic predisposition for health problems.
And even if one day we are able to change the DNA in human embryos, scientists probably won’t recommend it any time soon. We don’t know enough about what genes do in our bodies to start modifying them. This could change in the future, but for now, geneticists discourage tinkering with an embryo’s DNA (even for simple cases).

Choosing Healthy Babies
Imagine that you and your partner both have relatives with cystic fibrosis (CF). In other words, both of you have a family history of CF.
This means that you both might be carriers for CF. Carriers do not have the disease, but they can pass it on to their children. If you and your partner are both carriers, then each of your children has a 25% chance of getting the disease.
If you want to make sure to have a baby that won’t get CF, the first step is for the two of you to receive genetic testing. If it turns out that both of you are carriers, right now the only way to ensure that your child doesn’t end up with CF is to use something called pre-implantation genetic diagnosis (PGD). This is where doctors look at the DNA of embryos and select those without the disease to grow into babies.
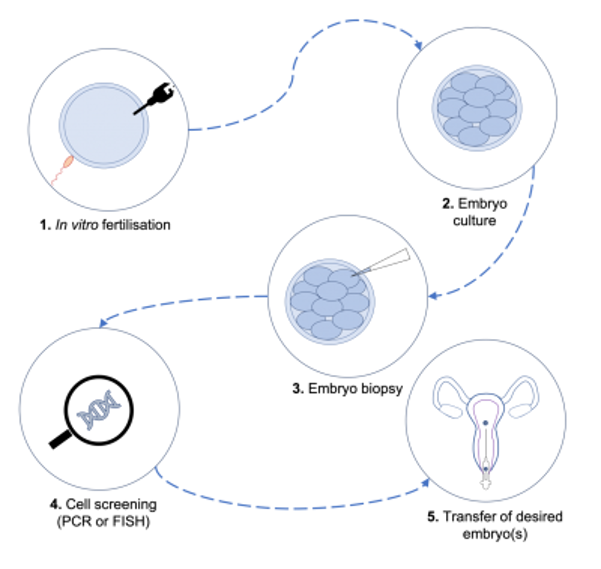
To use PGD, you must use in vitro fertilization (IVF) to conceive the baby. IVF is basically mixing eggs and sperm in a test tube to make embryos outside of the body.
The next step is to look at the DNA in the embryos to find the ones that did not get the CF-causing DNA from both of you. This is possible because the embryos are made outside the body.
The final step is to implant an embryo that won’t get CF in the uterus of the mom. Then you and your partner will have a baby without CF.
Right now, this is the only way that two carriers can be certain that they will not have a baby with CF. In the future, we might be able to change the embryo’s DNA instead to prevent CF. But we are not there yet.
Obstacles for Gene Splicing
You can imagine that there might be cases where all of the embryos will develop a genetic disease. In this case, you might want to change the DNA to have a healthy baby. This is much harder than it sounds.
There are a couple of different strategies to do this, both of which have problems. In the first, you add a new piece of DNA that inserts somewhere random in the cell’s DNA. In the second, you replace the bit of DNA that is broken with a bit that is not.
You have probably heard of the first approach: it is known as gene therapy and has been around for years. Gene therapy has only been used to treat diseases in fully grown babies, not to add new genes to embryos. Unfortunately, it has a whole lot of problems.
First off, our cells don’t like foreign DNA. They often shut it down or even spit it out.
And even if we get the DNA in a cell and it keeps working, it can cause unexpected problems. These usually happen because the new DNA inserts into the cell’s DNA in a random place.
When this happens, the new DNA can affect how nearby DNA works. This can cause cancer or other problems. In fact, in a famous set of experiments, a number of kids were cured of a disease but ended up with cancer!
Given all of these problems, it would be much better if scientists could simply fix broken DNA instead of injecting new DNA. Scientists are getting close to being able to do this but they aren’t quite there yet.
CRISPR-Cas9
Recently, scientists have developed a new tool to cut out DNA in a specific location and help a new piece of DNA insert there. This means they can replace a piece of DNA that causes a disease with one that doesn’t! It is based on a system called CRISPR-Cas9 that some bacteria use to fight off viruses.
Scientists have successfully changed DNA in mouse embryos using CRISPR-Cas9 and recently, it was even used in monkeys. But it is still too dangerous for use in people.
In the monkey study, scientists injected 186 embryos with CRISPR-Cas9 to remove a piece of DNA. Following injection, only 86 of those embryos were healthy enough to implant into surrogates, and only ten pregnancies took.1
Of those ten pregnancies, all of the monkeys born had only lost the DNA in some of their cells. If this system will ever be used in humans, it must become much better.
We would need a system that works every time in people. If it didn’t work, you would end up with a lot of “failed” embryos, which could raise ethical concerns since they would most likely be discarded. The monkey study shows that CRISPR-Cas9 is nowhere near as efficient as we would need it to be for use in humans.
In addition, these technologies are very new and we do not know yet how safe they would be in humans. We cannot be certain that the DNA will not be cut in multiple places, which could result in unforeseen problems (like cancer for example).
Gene Splicing: Risky Business
Obviously, people are ethically concerned by the idea of changing DNA to make “perfect” or “designer” babies. But there are more subtle concerns too.
Scientists are just beginning to understand what only a small fraction of our DNA does. Changes that might first look beneficial could in fact turn out to be harmful.
For example, people with a short version of a piece of DNA called the SERT gene (SERT-S) are at much higher risk of clinical depression.2 At first glance, it might appear beneficial to get rid of the short version entirely. However, more in depth studies show that environmental factors influence how this gene works.3
It is risky to assume that we fully understand what any bit of DNA is doing in our body.
It turns out that how you were raised influences how SERT-S affects depression. If you were abused as a child, then you are more likely to be depressed. But if you had a happy childhood, then SERT-S makes you less likely to be depressed. If a parent chose to remove SERT-S from their child’s DNA, they might reduce the likelihood that their child will have a happy life.
This is just one example showing that it is risky to assume that we fully understand what any bit of DNA is doing in our body. DNA that might be harmful in one environment could be beneficial in another. Scientists can’t predict the potential effects of changing DNA.
While technology is moving rapidly towards making changing DNA possible in humans, we should be cautious about how we use these new technologies. We must remember how little we know about how our DNA works.
Read More:
- Genetic Literacy Project: More on CRISPR-Cas9
- Scientific American: PGD: the slippery slope to designer babies

Author: Catherine Hartzell
When this answer was published in 2014, Catherine was a Ph.D. candidate in the Department of Immunology, studying calcium signaling and T-cell activation in Richard Lewis’ laboratory. Catherine wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
