
Why is GC content used to determine DNA melting temperature?
October 17, 2025

- Related Topics:
- DNA basics,
- Molecular biology,
- Biotechnology
A college student from Iran asks:
"I want to know why GC content is used to determine DNA melting temperature and not AT content?"
Imagine trying to pull apart two zippers - one with big metal teeth and the other with tiny plastic ones. Which would be harder to separate? It turns out that DNA works a bit like this! Scientists have found that G-C “teeth” hold on tighter than A-T ones, making it harder to unzip DNA that has a lot of Gs and Cs. That’s why the GC content of DNA is something researchers care about in the lab when they’re trying to tease apart two strands of DNA!
But let’s back up a bit. Why would we ever even want to unzip DNA? And what’s holding those two strands of DNA together in the first place?
DNA: The Spiral Code of Life
When you think of DNA, chances are you’re probably imagining that iconic double-helix structure which twists like a spiral staircase. Each step of the staircase is made up of pairs of chemical “letters” - A, T, G, and C. But these letters aren’t just symbols; they actually stand for nucleotides, which are the building blocks of DNA! Each nucleotide has a sugar, a phosphate, and a base (that’s the letter part!), with the bases pairing up in a very specific way: A always pairs with T, and G always pairs with C.

It’s precisely this pairing between bases that lets two strands of DNA snugly fit together, twisting into the double helix. But let’s now take a closer look at what exactly makes some pairs stick together more tightly than others - and why that matters when DNA “melts.”
Turning Up the Heat on DNA
DNA melting might sound like something out of a sci-fi movie, but it’s really just a way of talking about separating two strands of DNA. When heated, the bonds holding the strands together break, causing the DNA to unzip into single strands. Scientists use the melting temperature, or Tm, to denote the temperature at which half of a DNA sample separates. The higher the melting temperature, the harder it is to pull apart those two DNA strands!
So, what’s keeping the DNA double helix together in the first place? The answer is hydrogen bonds.
Tiny Bonds with a Big Job
You might have heard that opposites attract. Well, hydrogen bonds are certainly a testament to that! A hydrogen bond is a weak electrical attraction that forms when a hydrogen atom, which has a slight positive charge, is drawn towards an atom with a slight negative charge - usually oxygen or nitrogen.
In DNA, these bonds form between the nitrogen and oxygen atoms of one base and the hydrogen atoms of its complementary base on the opposite strand. But here’s the key: although adenine (A) pairs with thymine (T) through two hydrogen bonds, guanine (G) pairs with cytosine (C) through three. This difference arises from the specific arrangement of atoms in each base - G and C simply have more places where those hydrogen bonds can form.
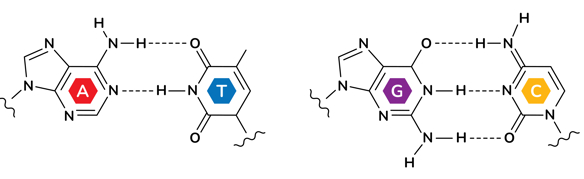
That one extra bond might seem tiny, but across an entire DNA molecule - which can contain millions of base pairs - it adds up to a major difference in stability. So the more G-C pairs a stretch of DNA has, the more total hydrogen bonds it contains and the harder it is to separate the two strands when heated.
GC Content: The Key to DNA Stability
This is where GC content comes in. Scientists measure the percentage of G and C bases in DNA to figure out how much heat it can withstand before melting. As you might expect, DNA with a higher GC content also generally has a higher melting temperature! In fact, for short DNA sequences, scientists have come up with a handy rule of thumb called the Wallace rule for approximating Tm:
Tm ≈ 2 x (number of AT pairs) + 4 x (number of GC pairs)
Notice how G-C pairs add about twice as much to the melting temperature compared to A-T pairs. That’s exactly why scientists focus on GC content when thinking about Tm - it just has a bigger impact on DNA stability.
Why should we care about melting temperatures?
Knowing the melting temperature of DNA is useful both in and out of the lab!
Scientists often use Tm values for procedures like PCR (polymerase chain reaction), which creates millions of copies of a specific DNA sequence. PCR is used in a wide variety of applications - for instance, some COVID-19 tests rely on amplifying tiny bits of viral DNA from a patient sample to detect an infection! PCR works by repeatedly heating and cooling DNA, so researchers need to know the right temperature to separate the strands without damaging them.
In nature, high GC content can help certain organisms survive in extreme environments. For example, some bacteria that live in hot springs have DNA with very high GC content - up to 70%!1 Those extra hydrogen bonds help keep their DNA from falling apart in the high heat. By contrast, bacteria living in colder environments often have lower GC contents since their DNA doesn’t need to be heat-resistant.2

So the next time you see those Gs and Cs in a DNA double-helix, you’ll know they’re not just random letters. Rather, they’re key to how DNA holds itself together across all life on Earth. Whether in a microbe bubbling away in a hot spring or in your own cells, those three tiny hydrogen bonds make all the difference in keeping the code of life from unraveling. Pretty incredible, right?

Author: Ronit Jain
When this article was published in 2025, Ronit was a graduate student in the Genetics Department at Stanford studying RNA-mediated gene regulatory processes. Ronit wrote this answer while participating in the Stanford at the Tech program.
 Skip Navigation
Skip Navigation
