How does telomerase work and how do cancers that don’t use telomerase keep dividing?
January 28, 2015

- Related Topics:
- Cancer,
- Aging,
- Chromosomes,
- Recombination
An undergraduate student from California asks:
"I learned in class that chromosomes get shorter every time a cell divides and that most cancers get around this with something called telomerase. How does telomerase work and how do cancers that don’t use telomerase keep dividing?"
Chromosomes do get shorter every time a cell divides. This is why cells can only divide so many times. Too many divisions and they lose key genes and die.
To protect its precious genes, each chromosome has something called a telomere at the end. This is kind of like padding. It is a repetitive bit of DNA that just makes the chromosome longer. That way some of this fairly unimportant DNA is lost in each division, instead of critically important genes.
This shortening is a real problem once we get to sperm and egg cells. These cells need to have full length chromosomes. Otherwise the eventual baby would have shortened chromosomes. They would get shorter and shorter each generation, until humans had no more DNA left!
Our cells get around this problem with telomerase. Telomerase is a special enzyme that adds DNA back to the ends of chromosomes. Sperm, eggs, embryonic stem cells and a few others make telomerase while the rest of our cells do not.
When a cell starts to make telomerase when it shouldn’t, bad things like cancer can happen. After all, a cancer cell is one that can keep dividing forever. In fact, most cancer cells make telomerase.
But as you point out, around 10-15% of cancer cells don’t. These cells have come up with a new way to keep their chromosomes intact. This process is called Alternative Lengthening of Telomeres (ALT).
As you might guess, ALT is a messy process and one that normal cells would never do. Normally if a cell needs a longer telomere, it turns to the enzyme telomerase.
Telomerase Adds DNA to the Ends of Chromosomes
As I said earlier, chromosomes lose a bit at each end each time they are copied. A cell prevents this slow decay of its DNA ends with telomerase.
Telomerase is an enzyme that adds short DNA sequences to the end of DNA. Unlike most enzymes, telomerase is more than just protein. It also has a bit of RNA too.
Telomerase uses this RNA molecule as a guide or template to add the right DNA to the ends of the chromosomes. In humans, it always adds the repeat sequence “TTAGGG.” It keeps doing this over and over and over.
In humans, there can be as many as 8000 copies of the repeat on the end of each chromosome! Together, these telomerase repeats make up a telomere.
Here’s a closer look at how the telomerase enzyme adds DNA to the end of a chromosome:
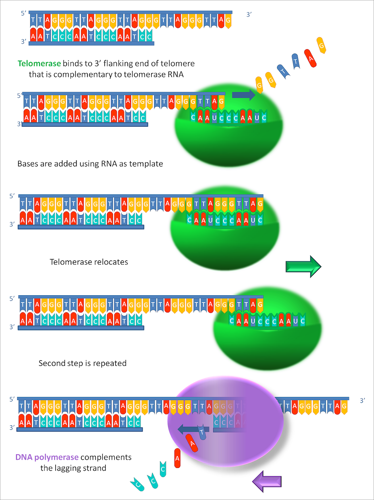
Each time a cell divides, its chromosomes lose a few of those repeats that telomerase added to their ends. Since in a healthy person only certain kinds of cells have telomerase turned on, this means that as people get older and their cells have divided many times, their telomeres get shorter and shorter. Eventually they lose too many repeats and their cells die.
At first it might seem weird that all our cells don’t keep making telomerase to keep their chromosomes from getting short when they divide. If telomerase were always active, a cell could keep dividing forever! Why wouldn’t we want that? Well, it turns out that having telomerase on all the time can sometimes have a nasty side effect: cancer.
Telomerase and Cancer
Many kinds of cancers have mutations that turn on telomerase all the time. Unlike in a normal cell, once cancer cells get telomerase on, they never turn it off. Instead the enzyme just keeps adding more and more repeats to the telomeres.
Now the cancer cell can keep dividing without losing DNA and genes at the ends of the chromosomes. Turning on telomerase is critical for keeping these cells going. Good for the cell, bad for the patient.
Telomerase activity has been found in almost all types of human cancer, although not all. Most cancers that do not have active telomerase have found other ways to maintain the length of their telomeres.
Alternative Lengthening of Telomeres (ALT)
About 10-15% of cancer cells use Alternative Lengthening of Telomeres (ALT), including some types of breast and bone cancers. But exactly how ALT works is not well understood.
We know they are able to keep the repeats at the chromosome ends, and we know that they do not have active telomerase. But how they pull it off seems to be pretty complicated. And it’s probably not the same in every different kind of cancer.
In cancer cells that use ALT (and most other cancers too), genes responsible for DNA repair end up mutated so they don’t do their job any more. Now these cells can’t fix their DNA, which means they build up mutations faster and faster.
Eventually the cell hits upon a combination of mutations that keeps its telomeres long. Usually this mutation turns on telomerase. But sometimes the cell hits upon a combination of mutations in a set of genes that keeps telomeres long without this enzyme. For lack of a better name, scientists have dubbed this ALT.
Currently, it is thought that ALT relies on homologous recombination to keep telomeres long enough for the cell to survive. In homologous recombination, DNA from one chromosome can be copied over to a different chromosome.
In healthy humans, homologous recombination only happens in very special circumstances, including during DNA repair and meiosis. (Learn more about homologous recombination here.)
However, some cancer cells have mutations that allow homologous recombination to happen whenever. Cancer cells can use this to copy long telomeres from one chromosome onto one with shorter telomeres like this:
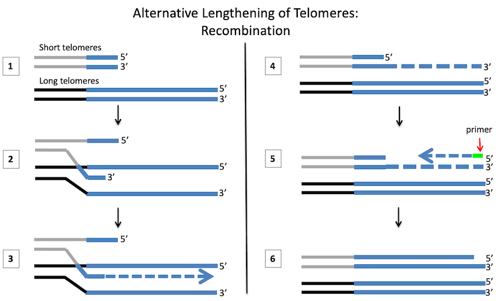
- Two chromosomes with different telomere lengths
- “Strand invasion”: An overhanging 3’ end “invades” the longer DNA strand of the other chromosome
- Polymerase extends the short chromosome, using the other as a template
- The two chromosomes separate
- Beginning with a primer sequence, polymerase fills in the opposite strand.
- The primer sequence is removed and any gaps are filled in.
Homologous recombination only happens between two DNA pieces that have some of the same sequence. Since the telomeres all have the same repeats, the ends of any two chromosomes have a lot of identical sequences.
During recombination, the ends of two different chromosomes pair up (step 1). Then one strand of the shorter telomere “invades” the longer telomere (step 2). Again telomeres are able to do this because they all have lots of copies of the same repeat sequence. These repeat sequences on the different chromosomes can then base pair and stick together.
Next, a DNA polymerase adds bases to the end of the short telomere, using the long telomere as a template (step 3). After the long telomere has been copied, the different chromosomes separate (step 4). Now that half of the original DNA piece has been extended, the strand with the newly copied telomere can be used as a template to fill in the other half (step 5). At the end of this, the telomere is much longer than it used to be.
By using homologous recombination, cancer cells are able to keep their telomeres long without needing telomerase at all!
Read More:
- Amoeba Sisters: Video on DNA Replication
- Utah Genetics: Detailed look at telomeres
- Telomeres and cell aging
- Where there’s some telomere sequence in the middle of Chromosome 2
- Read about Elizabeth Blackburn, who won a Nobel Prize for her work on telomeres and telomerase!

Author: Abbey Thompson
When this answer was published in 2015, Abbey was a Ph.D. candidate in the Department of Genetics, studying the molecular basis of evolution in David Kingsley’s laboratory. She wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
