
How do people get Friedreich’s ataxia and who is most likely to get it?
August 12, 2015

- Related Topics:
- Genetic conditions,
- Autosomal recessive inheritance,
- Carrier,
- Dominant and recessive
A high school student from Denmark asks:
“How do people get Friedreich's ataxia and who is most likely to get it?”
Friedreich’s ataxia is one of those diseases that can seem to pop up out of nowhere. Everyone is fine for as long as anyone can remember and then, suddenly, there it is. Someone in the family starts to have movement problems because of nerve damage.
The reason this can happen is because carriers of the disease show no symptoms. They don’t have Friedreich’s ataxia but one of their children can end up with it if the other parent is a carrier too. Each child in this case has a 1 in 4 chance of getting the disease.
At the gene level, people get Friedreich’s ataxia because they inherited a nonworking copy of the FXN gene from EACH of their parents. They have no working FXN gene and so develop the disease.
Carriers don’t have the disease because they have one working and one nonworking copy of this gene. They do not have Friedreich’s ataxia but they can pass their nonworking copy down to their children. If both parents do, then the child will have no working copies of the gene and so have the disease.
Diseases like Friedreich’s ataxia are called autosomal recessive diseases. And as you’ll see below, the 1 in 4 number comes from simple statistics.

Two Broken Copies of the FXN Gene Causes Friedreich’s Ataxia
Our DNA has a collection of genes that each plays a part in making us who we are. Each gene has the instructions for making/running one small part of us.
For example, we have a gene that lets us digest the sugar in milk, lactose, as an adult. Another gene plays a part in deciding whether or not we’ll have brown eyes. And so on for all 20,000 or 25,000 of them.
The FXN gene plays a role in the mitochondria, where cells get their energy. When there aren’t any functioning FXN genes, the mitochondria can’t make enough energy for the cell.
This lack of energy causes the cell to go into a state of stress and so it doesn’t function fully. The cells that are most affected are some nerve cells and muscle cells in the brain and heart.
We all have the same basic genes that help us grow up and survive. What makes each of us unique is that there are MANY different versions of the same genes (like blue versus green versus brown eyes), and that we have two versions of each gene – one we get from our mom and one we get from our dad (click here for exceptions to this rule).
The FXN gene is no exception. We each have two copies of the FXN gene, one of which we got from our mom and another from our dad.
As long as we have at least one working copy of the FXN gene, we will not get Friedreich’s ataxia because the working copy is enough for the body to work just fine. So, people might have the nonworking version of the gene and not even know it. Like we mentioned above, those people are “carriers.”
Two Carriers Have a 1 in 4 Chance to Pass on Their Nonworking Genes
When someone has only one working copy of the FXN gene, they are called carriers. These carriers give one copy to each of their children, but they don’t get to pick which copy. This means that each child of a carrier has a 1 in 2 chance of getting a parent’s nonworking copy.
If the carrier’s partner isn’t a carrier, then it is very unlikely the child will end up with Friedreich’s ataxia. But there is still a chance the child will be a carrier. So in this case, each child has a 1 in 2 chance of being a carrier and no chance of getting Friedreich’s ataxia.
However, each child of two carriers has a 1 in 4 chance of having Friedreich’s ataxia. This is because each child has a 1 in 2 chance of getting a nonworking gene from mom and a 1 in 2 chance of getting a nonworking gene from dad. When you have two chances like this you multiply the chances together. (1/2) X (1/2) = (1/4)
Math is a bit abstract so I will try to diagram things out to show where the 1 in 4 comes from. Here are two carrier parents:
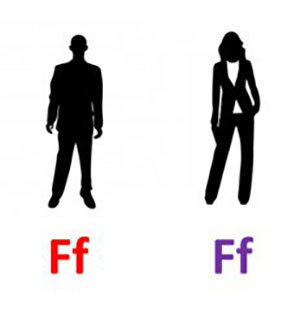

In this diagram, a working gene is shown with a capital F and a nonworking gene with a lowercase f. Geneticists usually label recessive gene versions with a lowercase letter.
I have also color-coded the genes to keep track of which gene copy came from which parent. Here, dad’s genes are red and mom’s are purple.
So neither the man nor the woman in the couple we show here has Friedreich’s ataxia because they have at least one working FXN gene (F). However, they are carriers because they each have one nonworking copy (f).
They will each give one of those copies to their child – but the version they give is totally random. So let’s say that Mom happened to give her nonworking FXN gene, f, and Dad gave his functional gene, F:
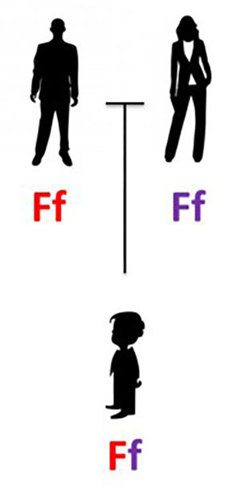

So this child is a carrier. He has one functional and one nonworking FXN gene.
BUT there are three other combinations that can result from the parents’ genes above, for a grand total of 4 possible combinations for the FXN gene. They are:


As you can see, 2 of the combinations, fF and Ff, result in carriers – they have one working and one nonfunctional gene. So there is a 2 in 4 (or 1 in 2) chance that the child will be a carrier, just like his or her parents.
However, there is a 1 in 4 chance that a child will get two working copies and be FF. Not only will that child not have the disease but he or she won’t even be a carrier. His or her children will not get Friedreich’s Ataxia no matter who they have kids with.
And there is a 1 in 4 chance a child will get an f from each parent and be ff. That child will have two copies of the nonworking gene and almost certainly end up with Friedreich’s ataxia.

Author: Camille Pataki
When this answer was published in 2015, Camille was a Ph.D. candidate in the Department of Biochemistry, studying lipid droplet protein targeting mechanisms in Ron Kopito’s laboratory. Camille wrote this answer while participating in the Stanford at The Tech program.
 Skip Navigation
Skip Navigation
